Using nonstick cookware to fry your bacon and eggs can make your life easier at that moment, but scientists believe there may be long-term consequences because the chemicals used to make it nonstick are so difficult to destroy. Perfluoroalkyl and polyfluoroalkyl substances — commonly known as PFAS and often called forever chemicals — are everywhere. PFAS, a suite of thousands of chemicals that have been around at least since the 1950s, are used for a wide variety of things, from the stain protectant on some of your clothing and linens to the food wrappers on your burgers.
The problem is that natural processes are ineffective at breaking PFAS down, so they accumulate in the environment and body, much like Styrofoam does in a landfill. Experts in science and industry are seeking ways to prevent PFAS contamination from occurring in the future, but they also aim to reduce what already exists in the world today.
It turns out that high-energy electron beams are excellent candidates for destroying PFAS in the environment. Researchers at the U.S. Department of Energy’s Fermi National Accelerator Laboratory, in collaboration with 3M, have successfully demonstrated that an electron beam can destroy the two most common types of PFAS in water — PFOA and PFOS.
“The electron beam is a promising technology to break down PFAS in large volumes of water that contain high concentrations of PFAS,” said Fermilab principal investigator Charlie Cooper.
The Fermilab team, which includes scientist Slavica Grdanovska, engineering physicist Yichen Ji and Cooper, used an electron beam accelerator at the laboratory for their testing. Used for proof-of-concept testing, the Accelerator Application Development and Demonstration accelerator, or A2D2, at the Illinois Accelerator Research Center on Fermilab’s campus is also available to industry, universities and other federal laboratories as a research tool.
“The fact that we were working with 3M, a world expert in PFAS, was really the first time that you had the experts on ionizing radiation, electron beam accelerators and PFAS working on the same project,” said Cooper.

Slavica Grdanovska presents water sample containers ready for testing at the A2D2 electron beam accelerator at Fermilab. Photo: Ryan Postel, Fermilab
Electron beams to the rescue
Conventional water treatment methods, such as reverse osmosis, granular activated carbon or ion exchange resin, do not destroy PFAS; they simply concentrate PFAS in a form which subsequently requires treatment or disposal. In some cases, conventional water treatment techniques can even make the environmental contamination worse.
In contrast, the electron beam actively destroys the forever chemicals and does so quickly, enabling a larger volume of water to be treated in the same amount of time as some other methods. PFAS molecules are difficult to break down because they contain a carbon-fluorine bond, which is very strong and the reason PFAS are commonly used in the chemical manufacturing industry. But the strength of that C-F bond is also the reason they don’t break down in nature. The electron beam, however, is very effective at breaking that C-F bond.
Electron beams could be used in pump-and-treat methods, a common groundwater treatment approach, or in a manufacturing facility, directly treating waste streams before they leave the facility.
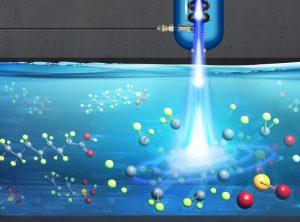
Illustration of an electron beam irradiating PFAS-contaminated water. Image: Samantha Koch, Fermilab
Demonstrating its effectiveness
The Fermilab team used PFAS-contaminated water samples provided by 3M that were sealed in gastight containers the size of a whiteboard marker. Each of the containers was made of borosilicate glass, which wouldn’t be significantly affected by exposure to electron beams, and an aluminum seal was crimped onto the glass with a piece of PFAS-free rubber between the aluminum and the glass. Great care was taken to ensure there were no PFAS in any of the materials used to house the samples. Fermilab irradiated these samples with the electron beam and shipped them back to 3M.
3M sampled both the headspace — the air at the top of the container — and the liquid to verify that the PFAS of concern had been destroyed without releasing hazardous products to the air.
PFAS are prevalent in many industries, and so is the human reliance on essential products that contain PFAS, such as computers and lithium-ion batteries. One of the most problematic PFAS-containing products in terms of environmental contamination has historically been aqueous film forming foam, or AFFF, which was used for firefighting at airports and in the military; it’s made of PFOA and PFOS. When you spray AFFF onto a liquid, it moves to the surface and extinguishes the fire by preventing oxygen from getting to it. But, when used it can seep into soil and groundwater. AFFF has been used in the United States and worldwide for decades, largely by the military and aviation industry. Recently, both government and industry started examining PFAS-free substitutes. Alternatives, however, do not exist in many applications and are hard to find or perform less effectively.
Although electron beams are very effective at breaking down entire suites of PFAS compounds, not every compound has been tested so far. The researchers found that all of the PFAS compounds the U.S. Environmental Protection Agency is currently considering regulating in drinking water were effectively destroyed by electron beam technology. But there may be types of PFAS an electron beam cannot destroy.
Research continues on several fronts to find alternatives to PFAS. At the same time, leaders in science and industry will continue to search for and enhance methods to eradicate these forever chemicals in the environment. Fermilab and its electron-beam technology stand at the forefront of this research.
This work is supported by the Office of Energy Efficiency and Renewable Energy of the U.S. Department of Energy Office of Science and 3M.
Fermi National Accelerator Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.